Purchasing a pharmaceutical water system is a major decision with long-term impacts. In this article we’ll break down the various factors influencing the capital cost of systems for producing pharmaceutical grade waters. By the end of this article, you will have a good idea of how much you should budget for your purified water (PW) system.
When producing purified water to meet USP, EP, BP or JP standards, there are 3 major items that influence the capital cost of a new system:
- The cost of the components that make up the system
- Engineering and design
- Project management, validation and GMP documentation costs
Before digging into the numbers below, please note that these prices are listed in 2021 Canadian Dollars. Pricing will vary by region and over time. At the time of writing, material prices were extremely volatile so we used pre-pandemic prices for our estimates.
Component Costs
When considering component costs you can mostly expect to get what you pay for. It’s important, though, to be realistic about where top quality components will actually make a difference and where they’ll just be more shiny.
Need a refresher on how water purification systems work? Juste ici.
Pre-treatment Equipment
Scale prevention, chlorine removal and sediment filtration are of utmost importance in the protection of downstream reverse osmosis systems. Some clients choose to impose sanitary design specifications on pre-treatment while others are willing to accept the very low risk of contamination associated with “industrial” equipment, requiring sanitary design only from the reverse osmosis machine downstream.
Dechlorination
Dechlorination is typically achieved either by injecting sodium metabisulfite (SMBS) or the use of a backwashable activated carbon filter. Some plants also require sanitary vessel, piping and valve design as well as steam-sanitizable carbon filters. In recent years, very high intensity ultraviolet irradiation (UV) has been used as an alternative method for dechlorination. visitez notre article à ce sujet.
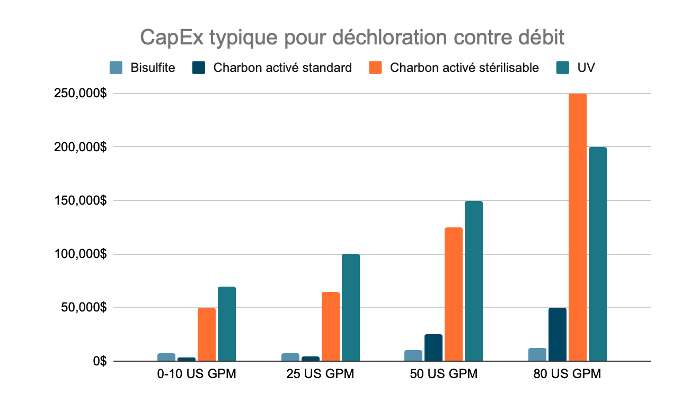
CapEx typique pour la déchloration, par débit
Il est important de noter que les membranes d’osmose inverse sont extrêmement sensibles au chlore et que le dosage du SMBS doit être étroitement contrôlé pour éviter des dommages irréversibles à la membrane. Un ajout excessif de SMBS conduit également à un encrassement organique prématuré des membranes d'osmose inverse.
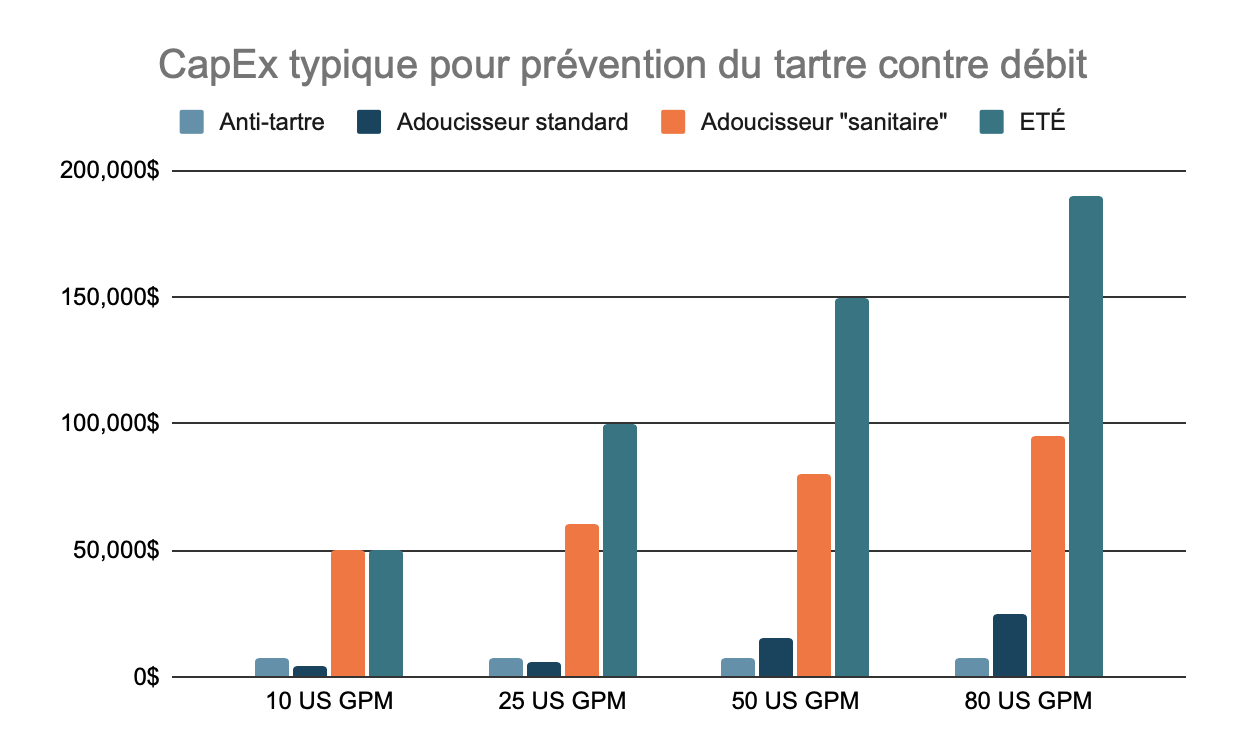
Scale Prevention
Similarly to chlorine removal, scale prevention protects reverse osmosis membranes. Depending on feed water hardness and other technical and economic considerations, systems use either chemical injection (antiscalant) or softeners to prevent hardness scaling. Some plants insist on sanitary vessels, piping and valve design for their softeners while others accept that non-sanitary pre-treatment poses very little risk for bacterial contamination of the purified water. Electrolytic scale reduction (ESR) is an emerging technology used in some pharmaceutical applications to replace softeners and antiscalant.
Typical CapEx for Scale Prevention, by Flow Rate
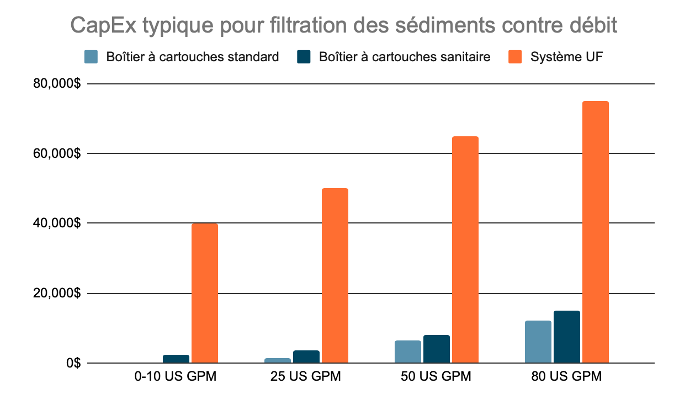
Sediment Filtration
Considérant que l’eau potable est un précurseur exigé par la majorité des pharmacopées pour produire de l’eau purifiée, la plupart des systèmes pharmaceutiques sont équipés de pré-filtres à cartouches pour protéger les membranes de l’osmose. Certains clients décident d’utiliser un système d’ultra-filtration (UF) pour réduire davantage le taux d’encrassement des membranes et éviter le changement trop fréquent des cartouches filtrantes. Certains systèmes utilisent aussi des filtres multi-médias ou des filtres au sable comme première étape de filtration.
Typical CapEx of Sediment Filtration, by Flow Rate
Reverse Osmosis System
At the heart of most pharmaceutical water purification systems is a reverse osmosis (RO) machine. While there are variations in design and construction of pharmaceutical RO equipment, there are fewer decisions to make that significantly impact costs. Below are typical cost ranges for the reverse osmosis equipment to be included in a pharmaceutical water system.
One significant factor affecting the cost of RO systems as their size increases is the requirement for pressure vessel registration on the housings. Different jurisdictions and companies have different rules. For example, many of our clients in Ontario (Canada) need to have Canadian Registration Numbers (CRN) for their RO housings. This can easily double or even triple the cost of the housings.
Another factor that significantly affects the cost of RO systems is choice of where to place the sanitary design boundary. Increasingly, we are seeing clients choose to design to sanitary standards only starting at the permeate (clean) side of the reverse osmosis system.
Typical CapEx of Reverse Osmosis, by Flow Rate
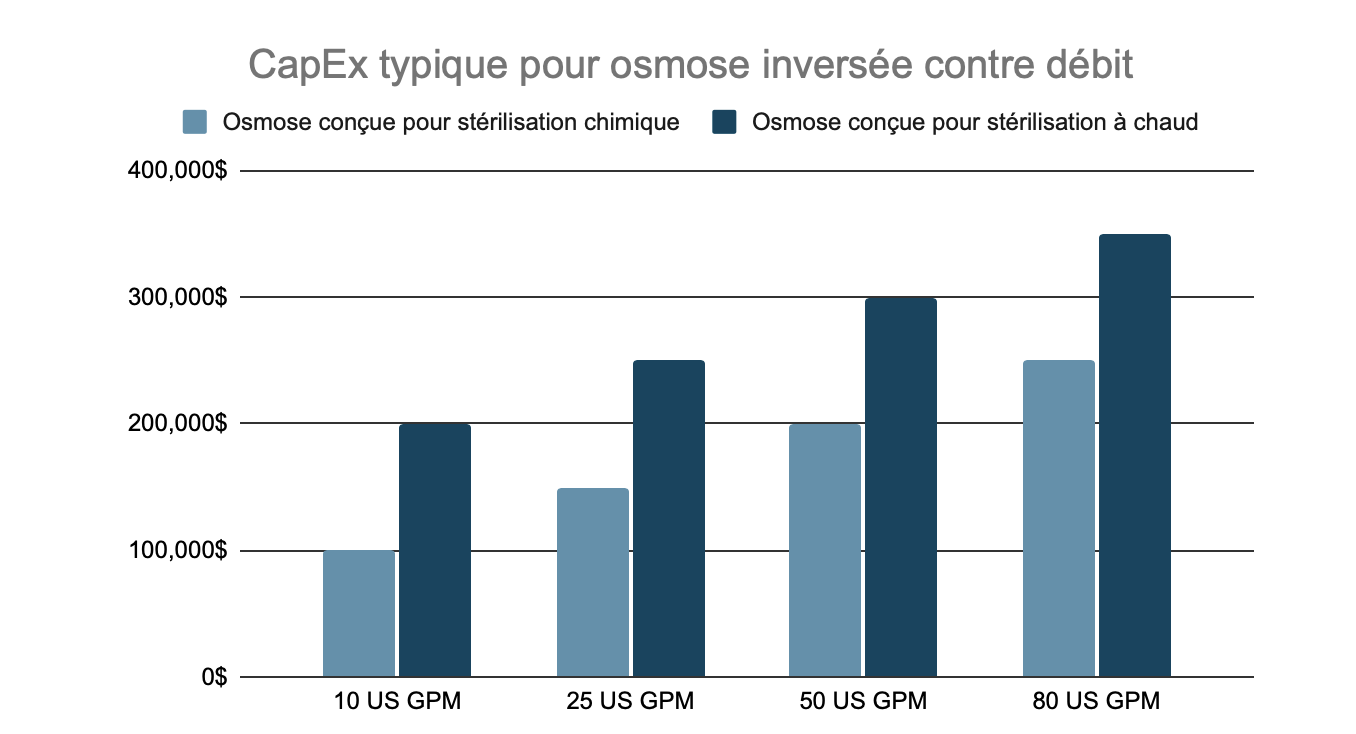
Pricing above is for reverse osmosis machines designed to be fully sanitary starting only at the permeate port. Semi-sanitary designs are more common and cost effective but often include a mix of tri-clamp, flanges and threaded connections. While bacteria control is of great importance in pharmaceutical water systems, most system manufacturers consider that everything before the RO permeate port will never be truly sanitary.
Polishing
Reverse osmosis is not typically enough to produce water that reliably passes the purified water or water for injection (WFI) standards established by the various pharmacopoeias worldwide. For this reason, most pharmaceutical water systems include a polishing step to further remove dissolved impurities. Technologies typically used for polishing are mixed-bed demineralizers, usually as service DI (SDI), electrodeionization (EDI) or second pass reverse osmosis, where applicable.
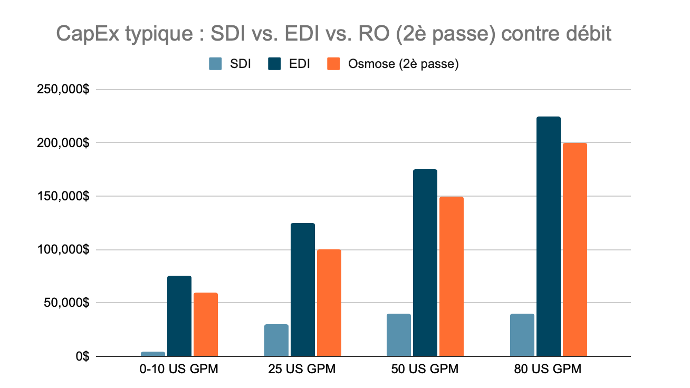
Typical CapEx of SDI vs EDI vs RO (2nd Pass), by Flow Rate
Final Filtration and UV Sterilization
Almost all well-designed pharmaceutical purified water systems include a final set of sub-micron (0.1-0.2 micron) final cartridge filters and an ultraviolet sterilizer as a last barrier to protect the point(s) of use. Some also include a UV for TOC destruction when total organic carbon levels are particularly critical. Below are the typical costs for these final steps:
Typical CapEx of Final Filtration, by Flow Rate

Typical CapEx for Final UV Sterilization, by Flow Rate
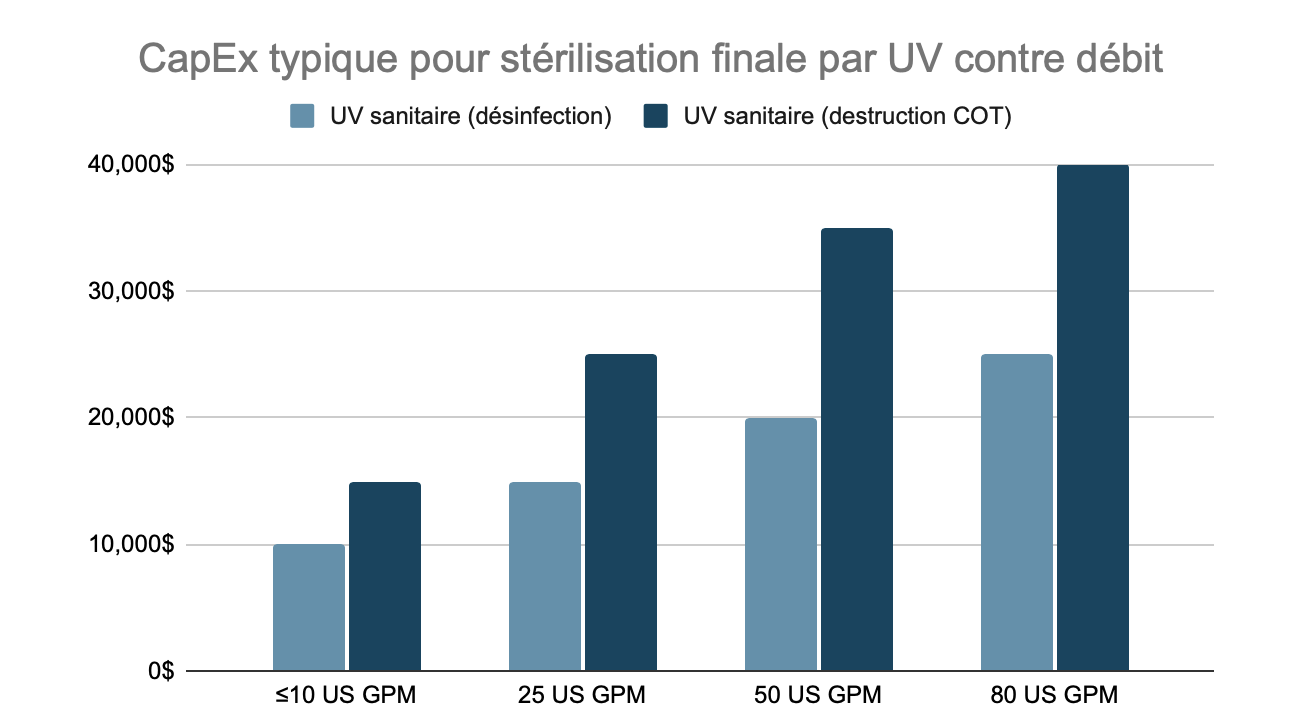
Note that the flow rates here are unrelated to the flow rate of water production at the RO outlet. The final filtration and sterilization equipment must be sized for the full flow of recirculation in the distribution loop, which is often significantly higher than the purified water production rate.
Atmospheric Tanks and Ozone Disinfection
La plupart des systèmes d’eau purifiée pharmaceutique incluent au moins un réservoir atmosphérique avec un évent filtré. Cette réserve d’eau permet au système de répondre à la demande sans avoir à surdimensionner l’équipement de production d’eau. Ces réservoirs sont conçus pour répondre aux exigences sanitaires telles que celles spécifiées dans ASME-BPE. En conséquence, ils représentent une partie significative de l’investissement en capital.
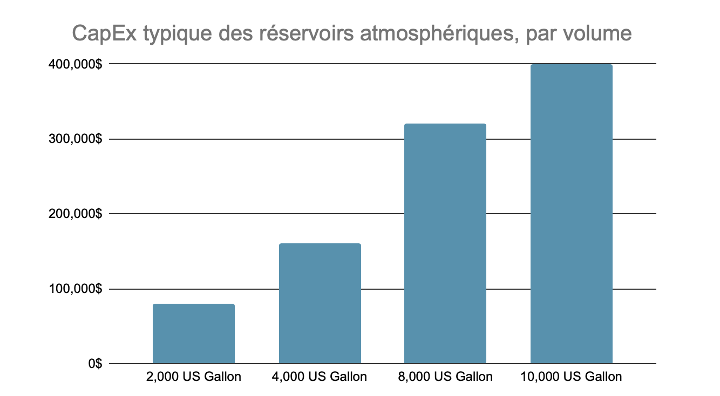
Le coût typique de ces réservoirs est d’environ 40 $ par gallon US (0,15 $ par m3). À titre d’exemple, un réservoir de 2 000 gallons US coûterait environ 80 000 $ tandis qu’un réservoir de 5 000 gallons US se vendrait 120 000 $.
Typical CapEx of Atmospheric Tanks, by Volume
Distribution Pumps
Flow and pressure requirements at the points of use, along with loop length and design (pressure loss) determine the size and price of distribution pumps required for a given plant. While applications vary quite a bit, a single sanitary distribution pump typically costs between $10,000 and $25,000 for flow rates up to 150 US GPM.
Due to the criticality of purified water to pharmaceutical production, most plants also opt for some type of redundancy. Should redundancy be desired, double your projected pump costs for a second pump (shelf spare) and add an additional 5 000 $ to 10 000 $ for piping and electrical components to make pump hot-swapping possible. Duplex alternating arrangements are to be avoided due to biological growth concerns around standing water.
Distribution Piping
Distribution piping costs depend on flow rate, length and the number of points of use. Typical pricing for a 2” loop is approximately $100 per installed foot while 1” loops run closer to $75 per installed foot.
Instrumentation and Controls
Contrary to most other components, costs for instrumentation and controls do not typically scale with the flow rate.
Typical control packages add about $20 000 to $50 000 to the total system cost, depending mostly on plant-specific standards. Simple machine-level PLCs will run closer to the low end of the budget range while more complex, plant-level PLCs such as AllenBradley ControlLogix will run at the higher end.
Instrumentation needs to be NIST-traceable, calibratable, traceable for materials in contact and tends to be premium-grade, especially downstream the RO permeate. A typical purified water instrumentation suite will cost between $50 000 and $150 000, including instruments for both the production system and the distribution loop. Costs depend on the types of instruments selected and the number of measurement points.
With the arrival of real-time, on-line bioburden analysis with instruments such as Mettler’s 7000RMS Microbial Detection Analyzer, some pharmaceutical producers choose to add another $150,000 to $200,000 of capital cost to significantly decrease the risk of lost production.
Skid Mounting and Interconnecting Piping
The costs of skid mounting and piping vary quite a bit depending on system design and application requirements. Below are some reference points to help you come to a budgetary estimate for your project.
Note that this pricing includes only the skid and interconnecting piping, not the treatment equipment mentioned on each row.
|
5GPM Purified Water Production skid and interconnecting piping, with skid-mounted carbon filter, softener, pre-filtration, RO and EDI |
$75,000 |
|
25-100GPM Distribution skid and interconnecting piping with skid-mounted pump, UV, final filtration and ozonation system |
$30,000 |
|
25GPM Purified Water Production skid and interconnecting piping for carbon filter, softeners, pre-filtration, RO and EDI (separate pre-treatment and RO-EDI skids due to overall size, SS carbon filter and valve nest, FRP softeners) |
$100,000 |
|
50GPM RO-EDI on single skid, including pre-filtration |
$60,000 |
|
50GPM pre-treatment skid and interconnecting piping for standard (non-sanitary) twin carbon filters and twin softeners |
$10,000 |
Engineering and Design Costs
As a general rule, pre-engineered systems cost less than custom engineered or adapted pharmaceutical water systems. This is especially true for smaller (low flow) systems because the engineering costs make up a larger portion of the total system cost.
Below is a reasonable estimate of the engineering and design hours required for a typical custom purified water system, based on our team’s experience:
|
Activity |
Approximate Number of Hours |
Approximate Hourly Rate ($/h) |
Total |
|
Process Engineering |
160 hours |
$150 |
$24,000 |
|
Detailed Mechanical Design |
300 hours |
$100 |
$30,000 |
|
Technical writing, O&M Manual |
80 hours |
$100 |
$8,000 |
|
Electrical Engineering & Controls |
160 hours |
$150 |
$24,000 |
|
|
|
Total |
$86,000 |
Rates for these professional services vary quite a bit but a good budgetary number to use is around $100 to $150 / hour depending on the service.
Project Management, QA/QC, Validation and GMP-Related Costs
Pharmaceutical clients typically require significant quality documentation throughout the system design, procurement, construction and testing phases. Each client has their own set of internal processes and policies and these have a direct impact on the cost of project management and quality management related to the purified water system. Below are some budgetary ranges you might expect for your project.
|
Activity |
Approximate Number of Hours |
Approximate Hourly Rate ($/h) |
Total |
|
Project Management |
150-300 hours |
$150 |
$22,500 to $45,000 |
|
Receipt checks |
100-200 hours |
$80 |
$8,000 to $16,000 |
|
Factory Acceptance Testing (FAT), including documentation |
150-450 hours |
$150 |
$22,500 to $67,500 |
|
IQ, OQ, PQ and MQ support |
120-240 hours |
$150 |
$18,000 to $36,000 |
|
|
|
Total |
$71,000 to $164,500 |
It’s important to note that these estimates are based on our experience in specifying, designing, building, testing and commissioning customized purified water systems. We are fairly conservative in our estimates and have seen a number of manufacturers offer support for validation lasting only two weeks (80 hours). Given the extreme variability in what clients require and what suppliers offer, we encourage you to get very clear on what type of QA and validation support you will require before getting quotes from suppliers.
Moreover, certain services and documentation, notably detailed receipt checks and detailed FAT protocols, are not often completed and supplied by manufacturers. This allows them to offer lower sale prices for the equipment. However, this also means that manufacturer-supplied QA documents cannot be leveraged effectively to reduce the time spent in IQ and OQ on site, which often carries a much steeper price tag for both OEM support and internal or third party validation support.
Conclusion
Les systèmes d’eau purifiée pharmaceutiques sont complexes et la variété disponible sur le marché est énorme. Nous espérons que cet article vous aura aidé à voir plus clair par rapport au budget que vous devriez prévoir pour l’achat de votre prochain système. Joignez-vous à la conversation en laissant un commentaire ci-dessous. Nous aimerions bien savoir comment on pourrait rendre nos articles plus utiles pour vous!